This is a main processes in the chemical and pharmaceutical industries to separate products from each other.
Whereas distillation takes advantage of different volatilities means different distributions of a product in the liquid and the gas phase the liquid/liquid extraction is based on different solubilities means different distributions of a product in 2 co-existing liquid phases.
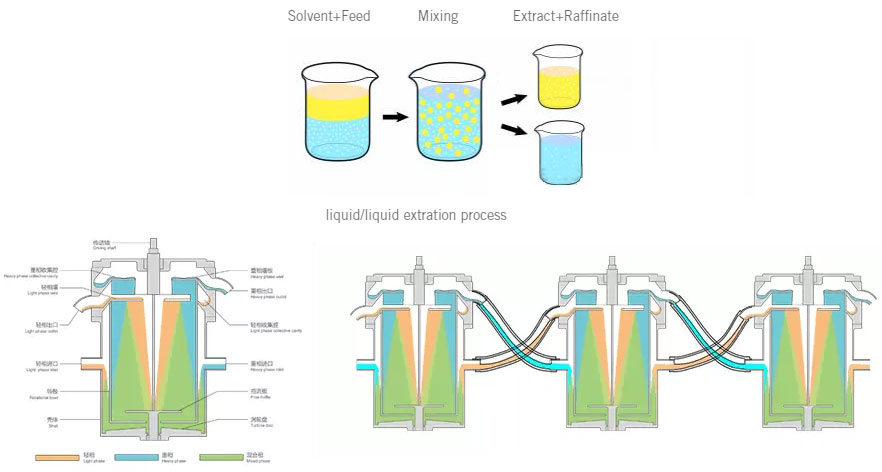
For the extraction of a product (white dots) out of the so called feed liquor (blue liquid with white dots) a suitable solvent (yellow liquid) has therefore to be found. The first step of an extraction process is mixing for an intensive contact of both liquid phases to enable the mass transfer of the product (white dots) from the (blue) feed liquor into the (yellow) solvent. After the extraction of the product the feed liquor is called raffinate (blue liquid with less white dots) whereas the solvent containing the product is called extract (yellow liquid with white dots). For the recovery of the product the solvent has to be separated in a subsequent from the product which is mostly done by distillation.
REQUIREMENTS FOR THE SOLVENT
The solvent will have to be checked for the following characteristics:
- Maximum solubility of the product in the solvent
- Minimum solubility of the solvent in the raffinate
- Minimum solubility of the feed liquid in the solvent
- Fast phase separation of the extract from the raffinate
- Easy separation of the product from the extract/solvent
With the right solvent there are numerous very different applications which can be realized advantageously by an extraction process as described below.
WHEN TO USE THE EXTRACTION PROCESS
The liquid/liquid extraction process is favorable for separations as for:
- products having similar volatilities
- products forming azeotropes
- products which would require high energy input in distillation
- products being temperature sensitive
- non-volatile products as e.g. metal salts

Separation of products with similar volatilities but different liquid/liquid phase distributions
The separation by distillation of mixtures with components having similar volatilities require high columns means high investment costs and high reflux ratios means high operating costs so that they may become economically not advantageous.
A very common mixture of a binary mixture with a low vapour/liquid separating factor is e.g. acetic acid in water. The acetic acid can be easily separated from water by extracting it with Methyl tert-butyl ether (MTBE) as solvent which can be evaporated easily.
- Acetic Acid /Water
- Aliphatic /Aromatic Compounds

Separation of products forming azeotropes
Products forming a homogenous azeotrope cannot be separated from each other by a conventional distillation above the azeotropic point. Sometimes this binary azeotropic mixture can be split by adding a specific third component or changing the operating pressure affecting the volatility of one product more than of the other. If this is not feasible a liquid/liquid extraction is the method of choice. Examples of such common mixtures are:
Aqueous systems
Tetrathydrofuran/Water
Pyridin/Water
Formic Acid /Water
Organic systems
Alkylchlorides/Alcohols as Dichloromethane/Ethanol
Ethylacetate/Ethanol
Separation of products which would require high energy input in distillation
There are many cases where aqueous effluents contain organic components having a higher boiling point than water. The separation of the organics by evaporation of the water would require a lot of energy and is hence in most cases not economic. The alternative is the extraction of the organic components with a solvent out off the water followed by an evaporation of the solvent to get the product. The evaporation heat of a solvent is mostly smaller than of water and the concentration of the product in the solvent is higher than it was in the water so that much less energy for the evaporation of the solvent is required than for the water of the feed liquor.
Such aqueous effluents are commonly either industrial waste waters or reaction mixtures.
Waste waters with high boiling organic compounds:
- Phenols, cresols and aniline or other aromatic derivates thereof
Reaction mixtures requiring the removal of organic compounds:
- Oxidation of organic products
- Production of caprolactam
- Syntheses of organic acids
Extraction Column for Fe/Ni Separation
Separation of temperature sensitive products
Another necessity for an extraction process can be the temperature sensitivity of the products themselves which can make a distillation process either impossible or economically disadvantageous.
Food, pharmaceutical and green chemistry bio-molcules as:
- Vitamins
- Penicilin
- Flavors and fragrances
Chemical Industries:
- Certains aldehydes and organic acids
Separation of non-volatile products as e.g. metal salts
The production or recovery of valuable metals requires often the separation of their metal ions from others in an aqueous solution. An option is the extraction of a metal ion if the metal ion forms a chelate complex in the organic phase leaving the other metal ions back in the aqueous phase.
Metals being recovered or purified are for example:
- Precious metals
- Rare earths
- Nickel/Cobalt
Tiei Extraction design and manufacture of CWL-M serial centrifuges extractors has laboratory scale centrifuges, pilot scale centrifuges and multistage production centrifuges.
Tiei Extraction focus on being Mixing And Separation Solution Provider