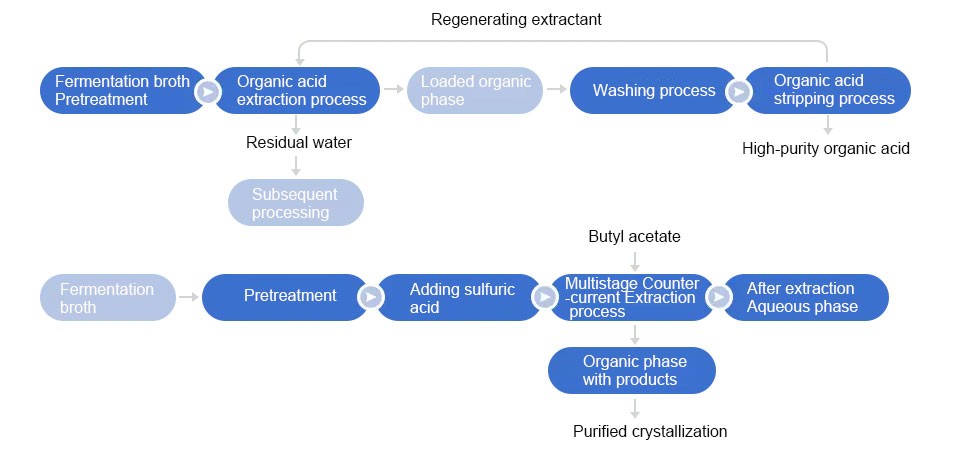
Liquid-liquid extraction involves the exchange of certain com-pounds between two solvents that are immiscible or only partially miscible. Liquid-liquid extraction is also very In an extraction, commonly used for washing an organic phase, for example to remove inorganic compounds, or to protonate or deprotonate bases or acids, respectively, so they become soluble in the aqueous phase. A very typical extraction flow diagram is shown below, where a reaction mixture is quenched with water, extracted (several times), washed with brine, dried, filtered and finally evaporated to yield a crude product or a pure product.
1.Extraction of neutral compounds. If the desired organic compound is neutral (i.e. is neither acidic nor basic), the extraction sequence usually involves simply extracting with an organic solvent several times.
2.Extraction of acidic compounds. If the desired compound is acidic, we can selectively deprotonate that compound by using an aqueous base. This will pull the deprotonated compound into the aqueous phase. Because very few organic compounds are soluble in water, we can discard the organic phase which now contains any byprod- ucts and/or unreacted starting materials. Acidification of the aque- ous phase can precipitate the desired product.
3.Extraction of basic compounds. If the product is basic, we can perform a sequence very similar to the acidic compound above. We can protonate the basic compound by using an aqueous acid, pulling the protonated compound into the aqueous phase and discarding the organic phase. Neutralizing the acidic phase will depro- tonate the basic compound, which may precipitate, or may require a second extraction with an organic solvent.
Tiei Extraction focus on being Liquid–Liquid extraction Mixing And Separation Solution Provider.