In the global zinc smelting industry, more than 80% of zinc production comes from the hydrometallurgical process. Its typical process includes oxidative roasting, acid leaching, high acid leaching, iron removal, purification and electrolysis. However, when processing high-iron zinc roasted sand, because it contains a large amount of zinc ferrite (ZnFe₂O₄), it not only increases the complexity of the high acid leaching process, but also significantly reduces the leaching rate of the valuable metal indium (usually less than 20%). Therefore, how to achieve efficient and comprehensive recovery of zinc and indium has become a key technical problem in the field of zinc smelting and solid waste resource utilization.
At present, the mainstream method for recovering zinc and indium from high-iron zinc roasted sand is to use pyrolysis, such as high-temperature volatilization in a rotary kiln to generate zinc oxide smoke, and then recover indium through a wet leaching-extraction process. However, this method has problems such as high energy consumption, high cost, and low indium recovery rate (generally less than 75%), which limits its widespread application in industry. At the same time, the behavior of zinc, iron, and indium in the leaching process is still unclear, which further affects process optimization and the improvement of metal recovery efficiency.
To solve the above problems, we proposed a technical route of "acid leaching-sulfuric acid roasting enhanced leaching-centrifugal extraction and separation", aiming to achieve efficient recovery and separation of zinc and indium.
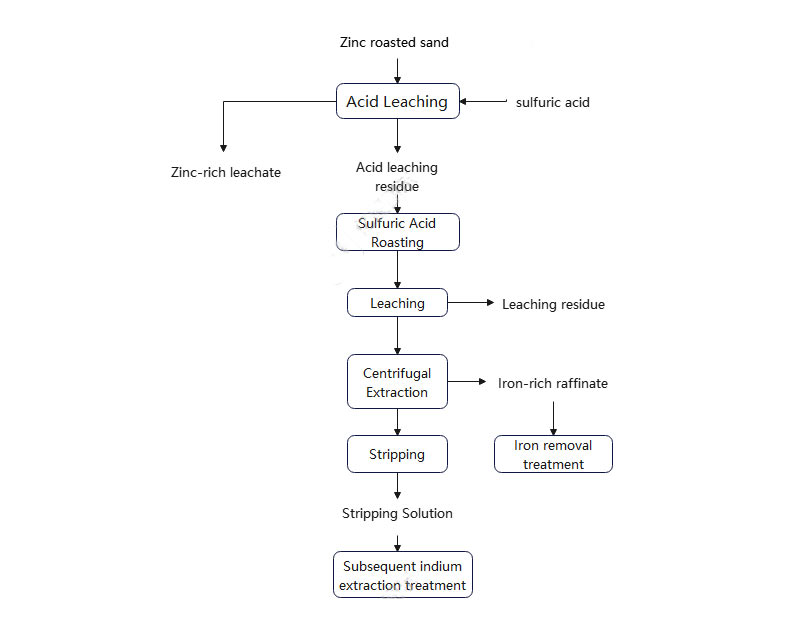
- Acid leaching: Initial enrichment of zinc and separation of indium slag. First, the high-iron zinc roasted sand is subjected to acid leaching treatment, so that the soluble zinc compounds therein are preferentially dissolved into the solution to form a zinc-rich leaching solution, while most of the indium remains in the leaching slag. The goal of this stage is to achieve the initial recovery of zinc and lay the foundation for the subsequent deep extraction of indium.
- Sulfuric acid roasting: Enhance the leaching of metals in indium-containing slag. For the leaching slag with high indium content after acid leaching, the sulfuric acid roasting process is used for thermochemical treatment. Under suitable temperature and atmosphere conditions, zinc ferrite can be decomposed and converted into soluble zinc sulfate, and indium is also converted into soluble sulfate, thereby significantly improving its leaching rate in the subsequent water leaching process. This process can not only effectively destroy the structure of zinc ferrite, but also improve the occurrence state of indium and enhance its release ability in the leachate.
- Water leaching of indium: The material obtained by sulphation roasting of a high indium concentration solution is then dissolved into the solution by water leaching or dilute acid leaching to obtain an indium-rich leaching solution. This liquid phase system provides a good basis for further metal separation and purification.
- Centrifugal extraction: To achieve efficient separation of indium from zinc and iron, in the traditional solvent extraction process, the distribution ratio of indium and iron in the two phases is similar, making it difficult to effectively separate the two. The centrifugal extractor technology uses the strong centrifugal force field generated by high-speed rotation to enhance the mass transfer efficiency of the two phases. At the same time, combined with the difference in mass transfer rates of different metal ions in the organic phase and the aqueous phase, effective separation of indium from zinc and iron can be achieved. By selecting a suitable extractant (such as P204, P507, etc.), selective extraction of indium can be completed in a short time, while zinc and iron are mainly retained in the aqueous phase, thereby achieving the goal of efficient metal separation. After stripping and subsequent purification, a high-purity indium product can be obtained.
Email: sales@tieiextraction.com
Whatsapp: +86 19069612820